Archives
Since at least prospective cohort studies have
Since 2005, at least 14 prospective cohort studies have been conducted to clarify inconsistencies in the field, ten of which suggested a direct correlation between antibiotic use and atopy or wheezing later in life [51–60], three found no association [61–63], and one found positive association only in the case of macrolides but not cephalosporins, ampicillin, penicillin, trimethoprim, or wide-spectrum filipin [64].
One of the problems that may result in failure to identify a statistically significant correlation, is a small sample size. Of note, all prospective studies using large cohort groups (>10000 subjects) mentioned in this review suggested a positive correlation between antibiotic use and atopy [47,51,53,54,57,59]. Additionally, a recent nation-wide population-based study in Sweden, taking into account 493785 children, identified a strong positive association between antibiotic use and the development of asthma, with the effects most pronounced during the first 6 months of life and gradually decreasing when antibiotic treatment was initiated later in life [57].
A phenomenon that could be perceived as a counterpart to antibiotic treatment is the exposure to diverse microbial communities, such as those evident in rural areas. Numerous epidemiological studies link exposure to farming environments with protection against allergies [65]. This ‘farm effect’ is in line with the ‘biodiversity hypothesis’, which points to an urbanization-induced decline in the variability of living species, including the microbiota, and, consequently, an increase in allergy [66–68]. Various factors were proposed to be responsible for the observed associations, from which contact with livestock and consumption of unpasteurized cow’s milk appear to be most important [69–73]. Future studies are needed to further delineate these protective effects and unravel the mechanisms they trigger in the host, which will complement studies on the influence of antibiotics, and contribute to the design of novel strategies to combat allergy and asthma.
Antibiotic Treatment and Allergic Inflammation in Animal Models
It is well documented that GF mice are more prone to mount Th2 responses [17] and are more susceptible to allergic models of diseases [74–76]. These data suggest an ability of microbiota to educate the immune system and prevent the induction of unnecessary responses.
Notably, this appears to be of major importance early in life since recolonization of GF mice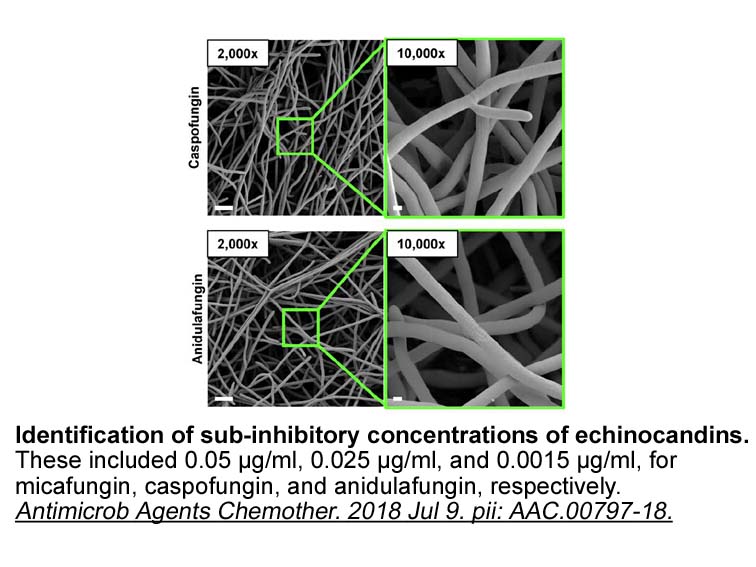 during the first 5 weeks after birth was shown to be sufficient to reduce IgE levels to that of specific pathogen-free (SPF) mice, while no effect was observed when mice were recolonized after 12 weeks of age [75]. The concept that there is a time frame after birth when microbial colonization is critical is often referred to as the ‘window of opportunity’ and has been extensively covered elsewhere [77–83].
These data match the observations made in epidemiological studies and suggest that antibiotic treatment of mice, especially early in life, exacerbates subsequently induced allergic inflammation. Unfortunately, only a handful of reports have addressed this notion. Bashir and colleagues reported that a 1-week antibiotic treatment of mice beginning at the age of 2 weeks rendered them more susceptible to peanut allergy, with elevated levels of Ara h 1-specific IgE, Th2 cytokines, and the overall systemic anaphylaxis score [84]. In two subsequent studies by Noverr et al., cefoperazone treatment followed by oral gavage with Candida albicans resulted in exaggerated Aspergillus fumigatus[85] and ovalbumin (OVA)-induced [86] allergic airway inflammation, although no firm conclusion was reached due to the absence of antibiotic-only control groups. Nevertheless, these experiments implicated a deregulated interplay between commensal bacteria and fungi as a possible driver of atopic diseases. This notion was later supported by Kim and colleagues, who observed outgrowth of Candida parapsilosis upon antibiotic treatment and correlated its loads with the increased severity of papain-induced airway inflammation [87]. The role of fungal dysbiosis in driving exaggerated allergic responses was further confirmed by Wheeler and coworkers, who treated mice with the antifungal drugs, fluconazole or amphotericin-B, followed by the induction of allergic airway inflammation. Interestingly, antifungal treatment did not result in complete eradication of fungal species, but rather in an alteration of their composition, with elevated levels of Aspergillus amstelodami, Epicoccum nigrum, and Wallemia sebi. Mice undergoing the treatment displayed an exacerbated allergic response, with higher levels of eosinophils and conventional DCs in the bronchoalveolar lavage fluid (BALF), increased levels of total IgE and HDM-specific IgG1 in the serum, as well as an elevated frequency of Th2 cells in mediastinal lymph nodes. Importantly, administration of the mix of A. amstelodami, E. nigrum, and W. sebi into naïve mice followed by allergen administration led to the exacerbation of the allergic response, recapitulating the results obtained with the use of antifungal drugs [88].
during the first 5 weeks after birth was shown to be sufficient to reduce IgE levels to that of specific pathogen-free (SPF) mice, while no effect was observed when mice were recolonized after 12 weeks of age [75]. The concept that there is a time frame after birth when microbial colonization is critical is often referred to as the ‘window of opportunity’ and has been extensively covered elsewhere [77–83].
These data match the observations made in epidemiological studies and suggest that antibiotic treatment of mice, especially early in life, exacerbates subsequently induced allergic inflammation. Unfortunately, only a handful of reports have addressed this notion. Bashir and colleagues reported that a 1-week antibiotic treatment of mice beginning at the age of 2 weeks rendered them more susceptible to peanut allergy, with elevated levels of Ara h 1-specific IgE, Th2 cytokines, and the overall systemic anaphylaxis score [84]. In two subsequent studies by Noverr et al., cefoperazone treatment followed by oral gavage with Candida albicans resulted in exaggerated Aspergillus fumigatus[85] and ovalbumin (OVA)-induced [86] allergic airway inflammation, although no firm conclusion was reached due to the absence of antibiotic-only control groups. Nevertheless, these experiments implicated a deregulated interplay between commensal bacteria and fungi as a possible driver of atopic diseases. This notion was later supported by Kim and colleagues, who observed outgrowth of Candida parapsilosis upon antibiotic treatment and correlated its loads with the increased severity of papain-induced airway inflammation [87]. The role of fungal dysbiosis in driving exaggerated allergic responses was further confirmed by Wheeler and coworkers, who treated mice with the antifungal drugs, fluconazole or amphotericin-B, followed by the induction of allergic airway inflammation. Interestingly, antifungal treatment did not result in complete eradication of fungal species, but rather in an alteration of their composition, with elevated levels of Aspergillus amstelodami, Epicoccum nigrum, and Wallemia sebi. Mice undergoing the treatment displayed an exacerbated allergic response, with higher levels of eosinophils and conventional DCs in the bronchoalveolar lavage fluid (BALF), increased levels of total IgE and HDM-specific IgG1 in the serum, as well as an elevated frequency of Th2 cells in mediastinal lymph nodes. Importantly, administration of the mix of A. amstelodami, E. nigrum, and W. sebi into naïve mice followed by allergen administration led to the exacerbation of the allergic response, recapitulating the results obtained with the use of antifungal drugs [88].